Source from Kelvin A. Power’s Facebook on March 16, 2020
(Anti-viral Immune Responses)မှာ(Vitamin C)ဘယ်လောက်အရေးကြီးလဲဆိုတာလူတိုင်းဖတ်သင့်ပါတယ်။အထူးသဖြင့်၊ဗီတာမင်စီ(High Dose)သောက်ခိုင်းလို့ကျွန်တော့်ကိုဝိုင်းဆဲကြတဲ့ဆရာဝန်တွေအားလုံးအောက်ကသုတေသနကိုသေချာစွာဖတ်ကြည့်စေချင်ပါတယ်။
Vitamin C Is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection.
–
L-ascorbic acid (vitamin C) is one of the well-known anti-viral agents, especially to influenza virus. Since the in vivo anti-viral effect is still controversial, we investigated whether vitamin C could regulate influenza virus infection in vivo by using Gulo (-/-) mice, which cannot synthesize vitamin C like humans. First, we found that vitamin C-insufficient Gulo (-/-) mice expired within 1 week after intranasal inoculation of influenza virus (H3N2/Hongkong). Viral titers in the lung of vitamin C-insufficient Gulo (-/-) mice were definitely increased but production of anti-viral cytokine, interferon (IFN)-α/β, was decreased. On the contrary, the infiltration of inflammatory cells into the lung and production of pro-inflammatory cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-α/β, were increased in the lung. Taken together, vitamin C shows in vivo anti-viral immune responses at the early time of infection, especially against influenza virus, through increased production of IFN-α/β.
Keywords: Vitamin C, Anti-viral immune response, Influenza A virus, Gulo (-/-) mice
Vitamin C is known as an essential anti-oxidant (1,2) and enzymatic co-factor for physiological reactions such as hormone production, collagen synthesis (3) and immune potentiation (4-6). Naturally, an insufficiency of vitamin C leads to severe injuries to multiple organs, especially to the heart and brain, since they are both highly aerobic organs that produce more oxygen radicals. In fact, studies of in vivo effect on vitamin C are difficult since most animals, except human and some primate, are capable of synthesizing vitamin C endogenously (7). However, Gulo (-/-) mice were recently developed by the L-gulono-γ-lactone oxidase (Gulo) gene deletion like human, thus they should be supplied with dietary vitamin C (8). It already has been reported that vitamin C concentration was decreased by 10~15% in plasma of the Gulo (-/-) mice without supplementation of vitamin C for 2 weeks (8). We also reported that vitamin C level was remarkably decreased in the most organs in the Gulo (-/-) mice without supplementation of vitamin C for 3 weeks (9).
In addition, we found that numbers of T cell was decreased in the spleen of vitamin C-insufficient Gulo (-/-) mice (10). Even though it is thought that vitamin C shows its anti-viral or anti-tumor effects through the up-regulation of the activity of natural killer (NK) cells and tumor specific cytolytic T lymphocytes (CTLs), its related evidences in vivo are still unclear. The reason why it is impossible to investigate in vivo effect of vitamin C is that all of animals could synthesize vitamin C from glucose thorough the action of L-glunolactone-γ-oxidase (Gulo), as described above (7). However, we confirmed that vitamin C up-regulates NK cell activity through the regulation of activating/inhibitory receptors on the surface of NK cell (our unpublished data). Since it is commonly known that vitamin C and NK cells are closely related to the prevention of common cold and the flu (11-13), we evaluated in vivo anti-viral effect of vitamin C and its related mechanism in Gulo (-/-) mice against influenza virus (H3N2/Honkong/1/68). First, wild type, vitamin C-sufficient Gulo (-/-) mice and vitamin C-insufficient Gulo (-/-) mice were subjected to intranasal inoculation of 20 hemagglutination units (HAU) of influenza virus, and then their survival was monitored. Interestingly, we observed that vitamin C-insufficient Gulo (-/-) mice expired within 1 week, but all of wild type and vitamin C-sufficient Gulo (-/-) mice survived (Fig. 1B). However, the supplementation of vitamin C on a day after virus inoculation could not prevent the death of vitamin C-insufficient Gulo (-/-) mice (Fig. 1B). It suggests that a sufficient amount of vitamin C is needed to prevent in vivo pathogenesis of influenza virus. Also, considering that H3N2 influenza virus shows a good circulation in humans and pigs as well as a slow antigenic drift in swine (14), we believe that the antigenic divergence between human and swine influenza virus might be increased. Therefore, our results shown in Fig. 1 suggest that vitamin C may effectively prevent severe or fatal damages in humans by the infection of influenza virus as well. To clarify the underlying mechanisms on the survival by the presence of the sufficient amounts vitamin C in the mice, we examined the viral titers in the lung of each experimental group. As shown in Fig. 2, viral titer in the lung from vitamin C-insufficient Gulo (-/-) mice was 10 to 15-fold increased, when it was compared with viral titer in wild type and vitamin C-sufficient Gulo (-/-) mice. However, when Gulo (-/-) mice were supplemented with vitamin C after virus inoculation, we could not observe a definite suppression of viral replication. This provides the importance of the vitamin C concentration at the initial stage of influenza virus infection. That is to say, damages through the replication of influenza viruses can be effectively prevented, when vitamin C concentration is sufficiently high at the initial stage of viral infection. If it is insufficient, however, the pathogenesis of influenza virus could not be prevented.
Increase of mortality of vitamin C-insufficient Gulo (-/-) mice by the infection of influenza A virus. Twenty hemagglutination units (HAU) of influenza A virus (H3N2/1/68/HongKong) was intranasally inoculated into wild type (n=10), vitamin C-sufficient Gulo (-/-) mice (n=10) and vitamin C-insufficient Gulo (-/-) mice (n=10) was as described in Materials and Methods. And then the survival of mice was monitored for 7 days after virus inoculation. (A) Mice without H3N2 infection, (B) Mice with 20 HAU of H3N2 infection.
Increase of influenza A virus replication in the lung of vitamin C-insufficient Gulo (-/-) mice. To analyze the effect of vitamin C on the suppression of viral replication in the lung, the lungs were excised from sacrificed mice (n=10 per each group) and total RNA was purified from lung homogenate as described in Materials and Methods. The virus replication in the presence or absence of vitamin C was determined real time RT-PCR by using of specific primers for influenza virus M2 gene and β2m.
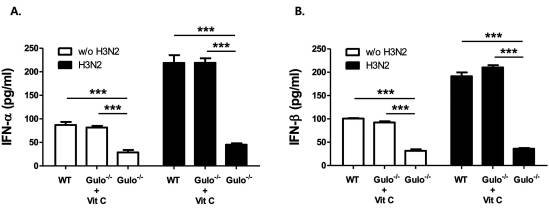
It is known that type I interferons (IFNs), IFN-α and -β, play an important role in prevention of viral pathogenesis as their amounts increase within 1 or 2 days after virus infection (15,16). We then measured the amounts of IFN-α and -β in bronchoalveolar (BAL) fluid and plasma in each mice after intranasal inoculation of influenza virus. As we expected, the levels of IFN-α and -β in BAL fluid and plasma from vitamin C-insufficient Gulo (-/-) mice were quiet lower than those in wild type and vitamin C-sufficient Gulo (-/-) mice (Fig. 3A and B). This result proves that vitamin C is an essential factor for the production of anti-vital immune response during the early phase of virus infection through the production of type I IFNs. The phosphorylation of signal transducers and activators of transcription (STATs) is the critical signaling process after the dimerization of its receptors, when type I IFNs are increased (17,18). Even though it is not presented this reports, we found the defects on the phosphorylation of STAT3 in T cells from Gulo (-/-) mice upon vitamin C insufficiency (10). Therefore, the activation of STATs in vitamin C-sufficient Gulo (-/-) mice upon virus infection should be further investigated.
Defect on the production of IFN-α/β vitamin C-insufficient Gulo (-/-) mice. The levels of IFN-α (A) and IFN-β (B) in BAL fluids from wild type (n=6), vitamin C-sufficient Gulo (-/-) mice (n=6) and vitamin C-insufficient Gulo (-/-) mice (n=6) were measured by ELISA as described in Materials and Methods, after 1 day of influenza A virus infection. Results are representative of three independent experiments and each performed in triplicates. Values are the mean±SD.
The defects on the production of type I IFNs followed by the activation of STATs are closely related to the inflammatory responses due to the failure of controlling virus replication at the initial stage of its infection (19). To investigate the inflammatory response in vitamin C-sufficient Gulo (-/-) mice by influenza virus infection, we compared the levels of pro-inflammatory cytokines, IL-1α/β and TNF-α in the BAL fluids from wild type, vitamin C-sufficient Gulo (-/-) mice and vitamin C-insufficient Gulo (-/-) mice after virus infection. As a result, the production of IL-IL-1α/β and TNF-α was definitely increased in vitamin C-insufficient Gulo (-/-) mice (Fig. 4A~C). At the same time, the infiltration of inflammatory cells into the lung is increased approximately 2.5-fold in vitamin C-insufficient Gulo (-/-) mice than in others (Fig. 4D). In our previous report regarding the preventive role of vitamin C on the pathogenesis of acute liver inflammation, we have observed that the increase of the infiltration of immune cells into the liver and slight increase of TNF-α production in vitamin C-insufficient Gulo (-/-) mice (10). And then the excessive inflammatory responses were occurred by the in vivo challenge of concanamycin A (10). Taken together, it seems that in vivo vitamin C insufficient status induces hyper-reactive immune responses against virus/bacterial infection or chemicals.
Increased production of IL-α/β and TNF-α in the lung of vitamin C-insufficient Gulo (-/-) mice by influenza A virus infection. The levels of IL-1α (A), IL-1β (B) and TNF-α (C) in BAL fluids obtained from wild type (n=6), vitamin C-sufficient Gulo (-/-) mice (n=6) and vitamin C-insufficient Gulo (-/-) mice (n=6) were measured by ELISA as described in Materials and Methods. Results are representative of three independent experiments and each performed in triplicates. Values are the mean±SD. (D) After centrifugation of BAL fluids, the numbers of infiltrated immune cells into the lung upon influenza A virus infection of in the presence or absence of vitamin C were counted under microscope.
It is generally known that plasma concentration of vitamin C in the mice is approximately 80~100µM. When the amount of vitamin C is present less than 20µM in the plasma, we called it as vitamin C insufficiency or “sub-scurvy” (9). Considering that systemic concentration of vitamin C in Gulo (-/-) mice at 3 weeks after vitamin C withdrawal was 10~20µM, we presented the effect of vitamin C insufficiency, not vitamin C deficiency.
—
In conclusion, vitamin C plays a critical role in vivo anti-viral immune responses against influenza virus through the increase of IFN-IL-1α/β production. Therefore, it might be possible that the maintaining sufficient levels of vitamin C in the plasma by the continuous uptake through the diet or supplement could effectively prevent in vivo pathogenesis of influenza virus at the initial stage of viral infection.
—